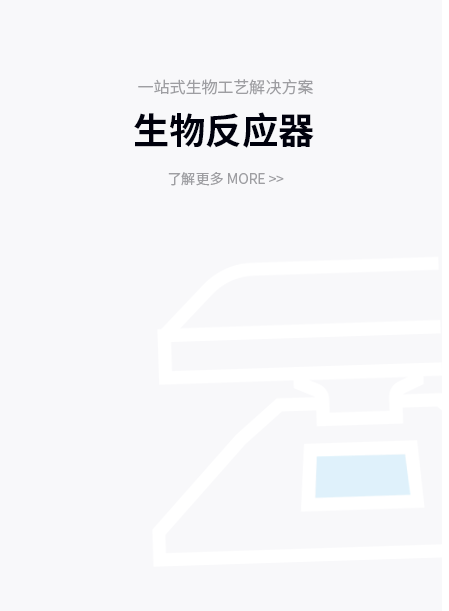
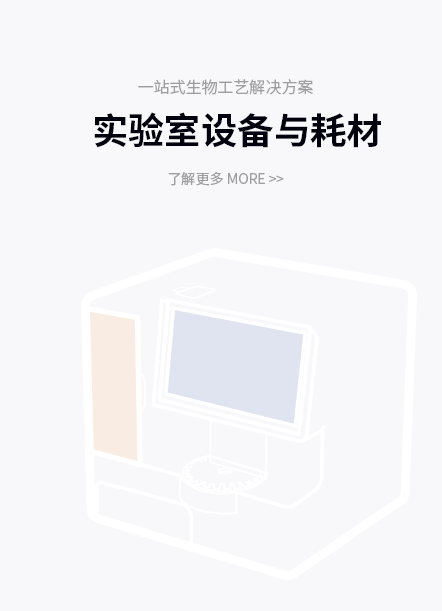
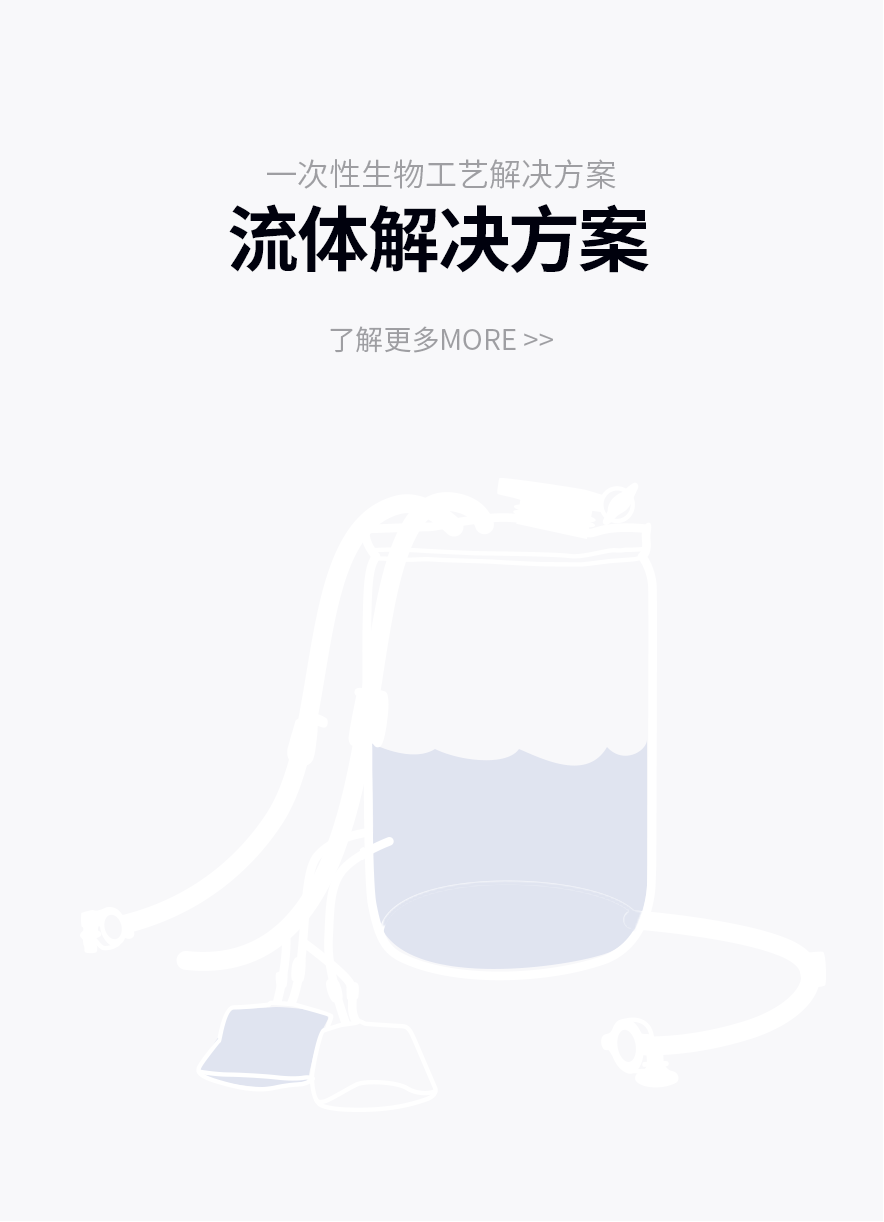
产品解决方案
提供针对不同应用场景的产品解决方案

了解更多
应用解决方案
提供针对不同应用场景的产品解决方案

了解更多
明星产品
提供针对不同应用场景的产品解决方案
公司动态
活动动态
技术专栏
解决方案
2025.03.31
公司动态
爱尔兰部长莅临上海,共同庆祝Branca Bunús和多宁生物建立战略合作伙伴关系
了解更多2025.03.31
公司动态
多宁生物荣膺2022【常春奖】年度健康服务平台
了解更多2025.03.31
公司动态
获选上海市“科技小巨人培育企业”,多宁生物构筑科技创新力
了解更多2025.03.31
公司动态
无锡多宁生产基地成功投产,持续加码供应链稳定
了解更多2025.03.30
公司动态
持续优化产业布局,多宁生物入股安赛尔
了解更多2025.03.31
公司动态
多宁生物与博腾生物达成战略合作 赋能CGT产业发展
了解更多2025.03.31
公司动态
多宁生物获选2022中国科学服务企业品牌100强
了解更多2025.03.31
公司动态
全球化再加码,多宁集团安拓思高压细胞破碎仪入驻康奈尔大学
了解更多2025.03.31
公司动态
多宁生物全球总部落户上海奉贤生物科技园,同步建设研发中心及技术应用中心
了解更多2025.03.31
公司动态
布局全新产品线!多宁生物与浚真生命科学达成细胞计数仪独家授权合作
了解更多2025.03.31
公司动态
无锡多宁顺利通过ISO9001:2015质量管理体系认证
了解更多2025.03.31
公司动态
联动共生 齐筑未来 多宁生物全球总部开工仪式顺利举行
了解更多2025.03.30
公司动态
多宁生物与凯德基诺达成战略合作 共拓基因治疗一体化解决方案
了解更多2025.03.31
公司动态
多宁生物入选“上海市专精特新中小企业”
了解更多2025.03.30
公司动态
多宁生物与中博瑞康达成战略合作,赋能CGT全生命周期发展
了解更多2025.03.31
公司动态
多宁生物获奉贤区“区级总部”认定及 “四新”经济示范企业
了解更多2025.03.31
公司动态
多宁生物并购普瑞流体 强化流体管理解决方案
了解更多2025.03.30
公司动态
精准分析提速生物药开发,多宁生物与优谱德达成战略合作
了解更多2025.03.31
公司动态
多宁生物与涛烜科学达成合作,助力经济、高效的抗体开发
了解更多2025.03.31
公司动态
多宁生物与巴斯夫达成深度合作,为生物工艺原材料稳健性保驾护航
了解更多2025.03.30
公司动态
多宁集团熙迈科技正式入驻浦东新区科技创新券平台
了解更多2025.03.30
公司动态
多宁集团亮黑科技顺利通过“科技小巨人”企业评定
了解更多2025.03.31
公司动态
多宁生物与Branca Bunús达成战略合作,推进新型转染试剂的市场应用
了解更多邮箱:marketing@duoningbio.com
地址:上海市松江区申田高科园民强路1525号30栋

官方公众号二维码

官方领英二维码