Lentivirus (LV) is a sub-type of retrovirus, with particle size of 80-120nm, consisting of a single-stranded RNA sequence, which is transcribed into DNA and can be integrated into the host genome, resulting in persistent infection. Most lentiviral vectors are derived from HIV-1 and retain the ability to integrate into the genome of infected cells. LV is very popular in clinical applications due to its ability to more efficiently transduce non-proliferating or slowly proliferating cells. Recently, multiple clinical trials have used third-generation self-inactivated LV to introduce genes into hematopoietic stem cells for the treatment of primary immunodeficiency or hemoglobinopathies. The most common application of LV is the delivery of introduced genes via chimeric antigen receptors (CARs) or cloned T cell receptors to generate immunity against tumors.
For the upstream production of lentivirus, although studies have shown that it is feasible to prepare stable lentiviral vector packaging/production cell lines, which has the potential to improve batch-to-batch consistency, ensure supply and reduce costs, because it can simplify the operation and supply chain of LV production, but often require a long time for cell line development, so the strategy of transient transfection of plasmid DNA based on HEK293T cells is still the main choice for large-scale production of lentiviral vectors. For cell culture, in the development phase, adherent culture is dominant given the need for only small batch volumes, the adherent properties of HEK293T cells and the easy availability of consumables. But as scale-up required higher and more consistent batch sizes, production shifted to suspension culture in rocking or stirred tank bioreactors, and the choice of media and supplements was directly related to production methods. The expansion of the cells is related to the activity and the success of the downstream process. Because the media can affect the stability of the vector and form the feed solution entering the downstream, it needs to be carefully optimized.

Compared with Adenovirus (AV) or Adeno Associated Virus (AAV), the greater challenge of LV downstream processing is that it is enveloped virus, and its inherent complexity and sensitivity require rapid processing, such as its sensitive to freeze-thaw cycles, salt, pH, shear and buffer osmotic pressure, and the particles have lower thermal stability, with a half-life of 7-8 hr at 37 °C. The downstream process of LV can also be divided into 3 main stages, including harvest and clarification; intermediate purification, which includes one or more concentration and purification steps; polishing, formulation and optionally sterile filtration.
LV is extracellular product that is released by the producer cells into the culture fluid, so the quality of the harvest fluid is highly dependent on cell viability, which determines the content of host cell-derived impurities. Clarification is aimed at removing cells and cellular debris, and depth filtration is an easy and economical option for this step, after optimization, it can achieve yields approaching 100%. In order to improve the overall biological safety of the final product and comply with regulatory standards, nuclease treatment is usually required to digest nucleic acids, which is also beneficial to reduce the viscosity of the feed liquid and facilitate subsequent steps. It is usually carried out after the clarification step, but it has also been reported carried out after a further reduction in volume at a more subsequent stage.
Chromatography is a key technology in the purification scheme, aiming at the removal of proteins, nucleic acids, low molecular weight impurities and lipids, etc. Anion exchange chromatography operated in bind-elute mode and multi-mode chromatography operated in flow-through mode have been reported, used alone or in combination for the purification of LV, but as mentioned earlier, due to the sensitivity of LV to conditions such as salt and pH, the conditions of the chromatography buffer should be carefully optimized. The use of immobilized metal affinity chromatography and size exclusion chromatography has also been reported, but generally limited to smaller scales. TFF is a fast and robust feed fluid concentration and buffer exchange technology. For LV, MWCO 750kD hollow fiber filter can be selected. A 0.22 μm filter can be used for the sterile filtration of the drug substrate before filling, but considering the large particle size of LV, it is reported that the loss of this step can reach 30-50%, and a strategy to reduce the loss is to perform it before the final TFF step, or use a closed system to achieve fully aseptic processing and skip this step.
None
 Hollow Fiber Filter Module
Hollow Fiber Filter Module 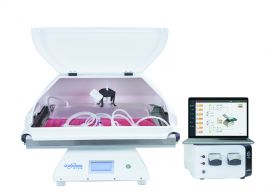 DuoWave® Rocking Single-use Bioreactor
DuoWave® Rocking Single-use Bioreactor  DuoBioX Pro Single-use Bioreactor
DuoBioX Pro Single-use Bioreactor  Transpro Series HEK293 Cell Culture Medium
Transpro Series HEK293 Cell Culture Medium  GlacierStore™ single-use freeze-thaw bag
GlacierStore™ single-use freeze-thaw bag  Mini DuoMix Mixing Bag/DuoMix Mixing Bag
Mini DuoMix Mixing Bag/DuoMix Mixing Bag  3D Liquid Storage Bag
3D Liquid Storage Bag  3D Foldable Cubic Liquid Storage Bag
3D Foldable Cubic Liquid Storage Bag  2D Liquid Storage Bag
2D Liquid Storage Bag  Peristaltic Pump BP701-XC45
Peristaltic Pump BP701-XC45  Batch Transfer Peristaltic Pump BP601-TH35
Batch Transfer Peristaltic Pump BP601-TH35  High-Precision Filling Peristaltic Pump BP521-PF246
High-Precision Filling Peristaltic Pump BP521-PF246 Copyright © Shanghai Duoning Biotechnology Co., Ltd. All Rights Reserved Sitemap | Technical Support: 
Message-